What Are Covalent Compounds?
Covalent compounds are substances that are formed when two or more different elements are joined by a covalent bond. A covalent bond is formed when two non-metal atoms share an electron. Atoms bond together in an attempt to become more stable. In general, atoms are more stable when they have the same amount of electrons as the nearest noble gas, and that usually means having eight electrons in their outer shell. In ionic bonds, this is accomplished by an atom with stronger electronegativity— the amount of pull an atom has to electrons— stealing electrons from those with lesser electronegativity. For covalent compounds neither atom is strong enough to steal the electrons, and so they share them.
There are two types of covalent bonds that can form covalent compounds: polar bonds and non-polar bonds. Polar bonds usually consist of different atoms unequally sharing electrons. This is often the result of a stronger electronegative atom drawing the electrons closer than a weaker atom. Since the electron spends most of its time closer to one atom than the other, the result is a covalent compound that has a partially negative end and a partially positive end.
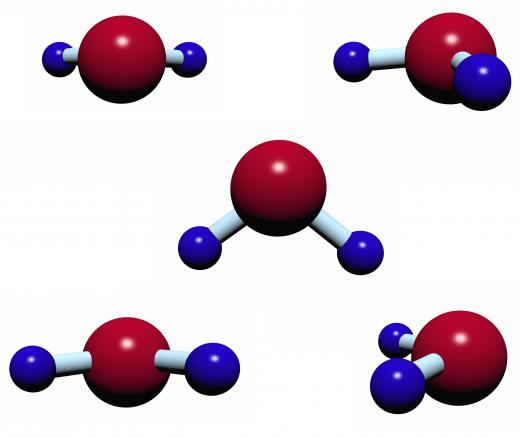
Non-polar covalent bonds are usually formed by two atoms sharing electrons equally. With these types of bonds, the electrons spend the same amount of time with each atom so there are no polar ends. A prime example of a polar molecule is water which has the chemical formula H2O. In this case, the oxygen atom attracts the electrons more to itself than the two hydrogen atoms, resulting in a covalent compound that is partially negative on the oxygen end and partially positive on the hydrogen end. An example of a non-polar molecule is the methane molecule (CH4) in which all atoms share their electrons equally.
In general, covalent compounds have a low melting and boiling point compared to ionic compounds. In addition, a substance made from covalent compounds tends not to be as hard as one made out of ionic compounds. These characteristics are a result of the ease of which the molecules are able to be separated. Though the atoms making up the molecules in a covalent compound are closely bonded, the individual molecules comprising the substance may have little hold on each other. For example, a person may have a hard time separating the hydrogen and oxygen in a water molecule, but boiling water— separating the molecules so that water turns from a liquid to a gas— is an easier task.
Other characteristics of most covalent compounds are their inability to dissolve in and their inability to conduct electricity in water. Lastly, covalent compounds tend to be flammable compared with ionic compounds. This flammability occurs because many covalent bonds tend to be comprised of carbon and hydrogen. Hydrogen and carbon can burn in the presence of heat and oxygen to form carbon dioxide and water in a reaction called combustion. As with all these properties, there are exceptions to the rule, for instance, covalent bonds that do not have carbon or hydrogen in their make up do not tend to combust.
AS FEATURED ON:
AS FEATURED ON:












Discussion Comments
Bohr received the Nobel Prize in Physics for his contribution to the word of science.
Bohr is most famous for his model of an atom. The Rutherford–Bohr model was a model of the hydrogen atom.
Although the model was a rather primitive and since determined obsolete, his model of the atom is still demonstrated in classrooms today. His model is the first step most take into the field of quantum mechanics.
Post your comments