What is a Spectroscope?
A spectroscope is a scientific instrument that splits light into its different wavelengths, which humans see as different colors. Violet has the shortest wavelength that people can see and red the longest. This instrument can also identify wavelengths that humans cannot see, such as infrared and ultraviolet radiation. Light usually contains a mixture of different wavelengths; by studying these, scientists can find out useful information, such as the chemical elements present at the source of the light. Spectroscopes are widely used in astronomy, chemistry, and other areas.
Types of Spectroscope and How They Work
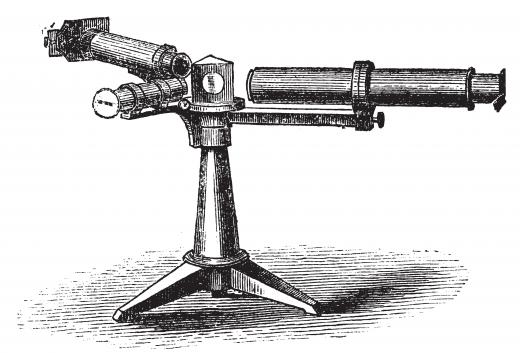
Joseph von Fraunhofer, a German optician, invented the spectroscope in 1814. In its early form, it used a lens to focus incoming light and a prism to split the light by refraction. Later, however, Fraunhofer replaced the prism with a device consisting of a number of narrow, parallel slits known as a diffraction grating. This spread out the different wavelengths of light by differing amounts and had the advantage of allowing the observer to actually measure the wavelengths, which was not possible using a prism. Fraunhofer used his spectroscopes to study light from a variety of sources, including flames, hot materials, and the Sun, planets and stars.
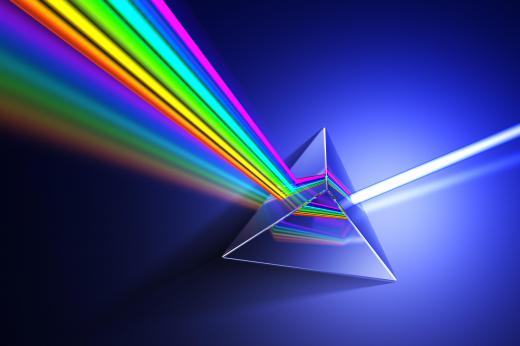
Modern spectroscopes come in a number of types, depending on their purpose. A simple hand-held device uses a small diffraction grating or prism and is easily portable. It is designed for use in the field, and can be used for identifying gemstones and minerals, for example. In astronomy, a spectroscope would normally be used with a telescope to analyze the light from distant, faint objects; these instruments tend to be heavy and bulky.

There are other instruments that do the same job as a spectroscope and work on the same principle. These differ mainly in the way the spectrum is recorded. A modern spectrometer produces a digital image of the spectrum, while a spectrophotometer records it electronically, and a spectrograph is a more general name for an instrument that produces and records a spectrum. These terms are sometimes used interchangeably and “spectroscope” may describe any of them.

Some devices can produce spectra for electromagnetic radiation with wavelengths beyond the limits of visible light. Since this radiation cannot be observed directly, the spectra need to be recorded by special detectors. These are used to study infrared and ultraviolet radiation.
An infrared spectroscope may use an adjustable monochromator to isolate each wavelength of interest in turn or, more commonly, an interferometer. This splits the incoming radiation into two beams. A moving mirror varies the length of one beam so that when they are brought together, they produce an interference pattern. Analysis of the pattern reveals the different wavelengths that are present. The interferometer method has the advantage of detecting all the wavelengths in one pass.
Types of Spectrum
Substances that emit light produce an emission spectrum. Hot, glowing solids — such as white-hot metal — emit light at all wavelengths and produce a continuous spectrum, where the colors merge into one another. Very hot gases, on the other hand, produce a line spectrum, which consists of colored lines against a dark background. This is because they emit light only at certain wavelengths, depending on the chemical elements that are present.
Each element has its own unique pattern of lines. Sodium, for example, produces strong lines in the yellow part of the spectrum. This can be seen by sprinkling salt (sodium chloride) into a flame, giving it a distinctive yellow color.
An absorption spectrum is produced when light at particular wavelengths is absorbed by a gas or liquid through which it passes. Each chemical element absorbs only certain specific wavelengths — the same ones that it emits as a hot gas — and so absorption spectra can also be used to identify elements. An absorption spectrum consists of dark lines against the bright background of a continuous spectrum.
The Sun produces a continuous spectrum with a number of dark absorption lines. The nuclear fusion process at the Sun’s core releases light at many wavelengths, but some of these are absorbed by various elements as the light travels to the surface, producing the dark lines. Scientists were able to determine the Sun’s chemical composition in this way. The element helium, which had never been seen on the Earth, was first identified by its absorption lines in the Sun’s spectrum.
Spectroscopy in Astronomy
Astronomers use spectroscopes to find out what elements are present in stars, in the atmospheres of planets, and in interstellar space. Stars have been found to differ in composition and can be classified according to their spectra. Spectroscopes have allowed researchers to find out what elements are present in the atmospheres of the other planets in the solar system. Astronomers may be able to analyze the atmospheres of exoplanets orbiting other stars; if oxygen was discovered, this would be a strong indication of life.
Examination of the light from other galaxies has revealed that, in most cases, the elements’ spectral lines are shifted toward the longer wavelength, red end of the spectrum, a phenomenon known as redshift. The most distant galaxies show the biggest redshifts, and most astronomers believe that this is because the universe is expanding. As the space between two objects increases, light traveling between them is stretched out, resulting in longer wavelengths.
The spectra of very distant objects, billions of light years away, are shifted beyond the range of visible light and into the infrared region. For this reason, infrared spectroscopy must be used to analyze them. Molecules produce infrared radiation at characteristic wavelengths when they vibrate or rotate. This method can, therefore, be used to identify the molecules present in gas clouds floating in interstellar space. Astronomers have discovered water, methane, and ammonia in gas clouds in this way.
Spectroscopy in Chemistry
In chemistry, spectroscopes can identify the elements present in a sample of material. Heating the sample strongly, such as in a flame, turns it into a hot, glowing gas that produces an emission line spectrum. Chemists can then examine this to identify the elements. This method led to the discovery of many of the elements in the periodic table. Alternatively, spectroscopy can capture the absorption spectrum of a liquid when a light is shone through it.
Chemists can use spectroscopy to identify chemical compounds as well as elements. Infrared spectroscopy is particularly useful in this respect, and it is often used in organic chemistry, biochemistry, and forensic chemistry.
AS FEATURED ON:
AS FEATURED ON:














Discussion Comments
it was very useful. thanks.
@ GenevaMech- Probably one of the best uses for spectrometry is in the mining industry. Geologists use a diffraction spectroscope to determine whether or not an ore is worth mining. Metal sulfides form underground will often form metal sulfates dissolved in solution (ground water). The metal ions then precipitate out of solution to form mineral deposits.
Geologists will sink drill holes and test the cross sections that they bring to the surface to see if the ore is of a high enough grade to mine. The ore is crushed and dissolved in acid, so it can be analyzed with a spectroscope. This may not be the exact procedure followed by mineral geologists, but the process of mining our mineral resources is something like this. I hope this is what you were looking for.
What are some other scenarios where scientists might want to use spectrometry in a real-world application?
I just completed a lab where we used an absorption spectroscope to determine the concentration of cobalt ions in solution. Essentially my lab partner and I used a colorimeter to measure the absorbance of 10 different known concentrations of Cobalt (II) Nitrate. Once we determined the absorbency of the ten different concentrations, we plotted them on a graph. Since the absorbency of the ten samples is linear and proportional, we were able to determine the absorbency, and thus the concentration, of the unknown sample. We only had a percent error of 3.2%, so this method was fairly accurate.
There are practical applications to this experiment. A farmer may want to know how much cobalt is in his soil so a lab may conduct a similar experiment to determine cobalt concentration in the soil.
Post your comments