What is Actinium?
 Mary McMahon
Mary McMahon
Actinium is a radioactive chemical element which is found in trace amounts in uranium ore. This element has a relatively short half life, and it is so radioactive that it has few industrial uses. The primary use for actinium is in scientific research. Consumers should rarely, if ever, interact with this element, which is just as well since it is extremely dangerous in the hands of people who are not experienced in handling radioactive materials.
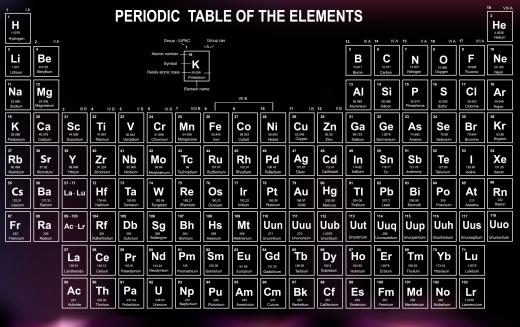
When this element is isolated, it proves to be a silvery color, and it will glow blue in the dark due to its radioactivity. The element shares a number of chemical properties with lanthanum, and the radioactivity makes it naturally extremely toxic. Actinium also produces a number of isotopes which have some research applications as well. On the periodic table of elements, you can find actinium by looking for the symbol Ac, and the element's atomic number is 89.
Credit for the discovery of actinium is typically given to Andre Debierne, a French chemist who isolated it from a uranium ore in 1899. Around the same time, radium and polonium were also isolated from uranium ore by Marie and Pierre Curie, showing that uranium held a few well guarded secrets. The name of the element is taken from the Greek aktin, which means “ray,” a reference to its radioactivity.

The primary users of actinium are scientific researchers, who utilize it as a source of neutrons in nuclear research. An isotope of actinium can also be used to bombard bismuth to produce some interesting reactions, and this isotope is also used in nuclear medicine. In addition to being found naturally, the element can also be produced synthetically, as was proved in 2000, when Australian researchers used a linear accelerator to produce a synthetic version.

Like other radioactive elements, actinuim is toxic, and it should be handled with care. Exposure to relatively small amounts can be very dangerous, and it should not be ingested. Researchers who work with the element typically use protective measures and monitor their radiation exposure to avoid levels which could cause radiation sickness or long term damage.
AS FEATURED ON:
AS FEATURED ON:










Discussion Comments
What are the uses of actinium? Please answer my question ASAP.
does it have any uses besides Thermoelectric power?
What are its uses?
does this element glow blue?
it was discovered by Andre Debierne in 1899.
it wasn't found. it was discovered.
who found the element actinium?
Post your comments