What is Calcium Sulfate?
 Mary McMahon
Mary McMahon
Calcium sulfate is a salt that occurs abundantly in the natural environment and also appears as a byproduct of some industrial processes. It is a compound of calcium, sulfur and oxygen, and in its purest form has the chemical formula CaSO4; this is known as anhydrous — water-free — calcium sulfate, or the mineral anhydrite. It also comes in a “hydrous” form, known as the mineral gypsum, which has the formula CaSO4.2H2O. The different forms have a wide range of applications, including building materials, desiccants, making casts for treatment of fractured limbs and creating works of art.
Properties

The hydrous compound has a low solubility in water that reaches a maximum at just over 100°F (38°C), but is decreases at lower or higher temperatures. Moderate heating drives off the greater part of the water, leaving the partially hydrated form as a white powder popularly known as plaster of Paris. The hydrated form can be recreated from this simply by adding water: initially a paste is formed, but this sets solid after a short time as the compound absorbs the water, giving out heat. Stronger heating drives away more water, leaving the anhydrous form, or anhydrite.
Occurrence and Production

Gypsum and anhydrite are common minerals that can occur in large deposits. They are generally clear or white, but can take on other colors due to impurities, and can be found as crystals or as layers of granular rock. Gypsum deposits were formed by the evaporation of inland seas many millions of years ago: layers of different minerals were laid down in a sequence, with those of lower solubility forming first as the water slowly disappeared. Where these deposits were subjected to heat, water was driven off, leaving anhydrite. In 2011, gypsum was found in a rock on Mars, indicating that water must have been present at some time in the past.

Naturally occurring gypsum is sometimes called alabaster, especially when used in sculpture, but this term is also applied to calcium carbonate. The two can be distinguished by treating with hydrochloric acid. Calcium carbonate reacts by producing bubbles of carbon dioxide, whereas gypsum is largely unaffected.
Most calcium sulfate comes from these deposits, which are mined in many parts of the world. Some is recovered as a byproduct of various industrial processes, especially those involving the treatment of calcium-containing rocks with sulfuric acid. An example is the production of phosphoric acid from calcium phosphate rock. In the laboratory, samples of the pure substance can be prepared by adding sulfuric acid to a solution of a soluble calcium compound; it is precipitated as a fine white powder due to its low solubility.
Uses
Gypsum is a relatively soft mineral that is easily carved into shapes, and because of this, has been widely used in sculpture, where it may be referred to as alabaster. Another common use for this form of calcium sulfate is in building. Sheets of gypsum are often used in the construction of internal walls in houses, due to its fire resistance — it remains relatively cool until most of the water content has been driven off, slowing the progress of a house fire. This mineral is also an essential ingredient in some forms of cement.

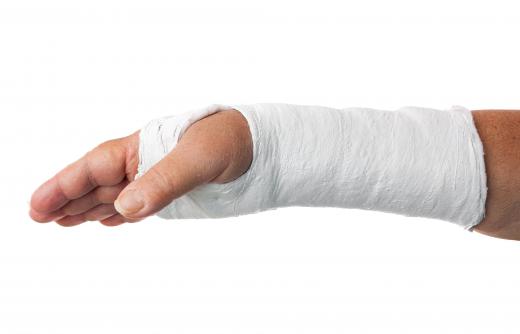
Plaster of Paris is widely used in medicine to make plaster casts for broken limbs, as well as in the arts, and in model making. Anhydrous calcium sulfate is used as a desiccant, to remove moisture where dry conditions are required — commercial forms may contain a compound that changes color in the presence of water to indicate when it has been exhausted. When this happens, it can be converted back to the anhydrous state by heating.
Calcium sulfate is also used in the food industry. It is often added to foods to give a firmer texture and sometimes as a source of calcium. The production of soy proteins such as tofu involves calcium sulfate, which is used as a coagulant to encourage the clumping together of small protein particles into larger pieces.
AS FEATURED ON:
AS FEATURED ON:












Discussion Comments
Where do Terra Alba and Snow White obtain their calcium sulfate from - or rather - what is it derived from?
Mother of child allergic to eggs and dairy products.
Why is calcium sulfate in Rumford's Non-GMO cornstarch? Isn't cornstarch a firming agent all on its own? (to make pudding, etc.). Good that they use Non-GMO corn to make their cornstarch (and hope to see the "Non-GMO Project Verified" label on it soon, so we can know their product is made with corn that's as close to being completely Non-GMO as is possible these days. I do not like it at all that there's something else (calcium sulfate) mixed in. Why?
plaster of Paris in your blood stream. nice. just what the doctor ordered.
it's in the tofu we buy. I think as a firming agent, like calcium chloride. --ryan
@dill1971: There are other foods that calcium sulfate is used in. In the baking industry, calcium sulfate is used in flour, cereal, yeast, bread conditioners, baking powder, and cake icing.
Also, in the brewing industry, calcium sulfate is used to make a smoother tasting beer.
Are there other calcium sulfate uses in other foods?
@anon97828: That is a very good question. It doesn’t sound very appetizing, does it? Calcium sulfate is used in the food industry as a reasonably cheap and FDA approved supplemental calcium source.
Two very popular calcium sulfate products are Terra Alba and Snow White filler. They are both approved fillers by the Food and Drug Administration’s Generally Recognized as Safe (GRAS) list.
Why is this stuff in Subway's bread?
Post your comments