What is Hexane?
Hexane is an organic compound made of carbon and hydrogen that is most commonly isolated as a byproduct of petroleum and crude oil refinement. At room temperature it is an odorless, colorless liquid, and it has many uses in industry. It is a very popular solvent, for instance, and is often used in industrial cleaners; it is also frequently used to extract oils from vegetables, particularly soybeans. Most vehicle-grade gasoline contains it, too. Though most experts say the compound is non-toxic and presents only low risks to humans and animals, there is still a great deal of controversy in many places when it comes to how often it is included, sometimes without full disclosure, in foods and consumer products.
Molecular Breakdown
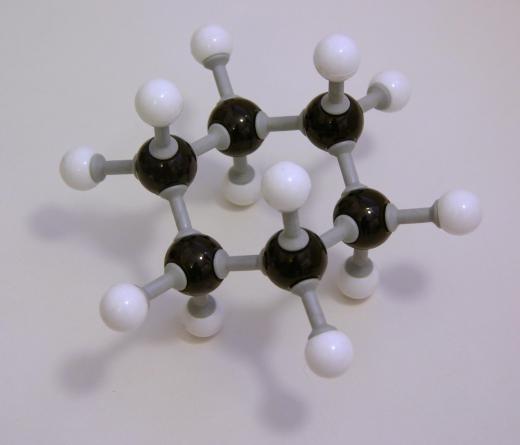
It is usually considered to be a relatively simple molecule. As the hex- prefix indicates, it has six carbon atoms, which are accompanied by 14 hydrogen atoms giving it the molecular formula C6H14. The carbons are chained in a row, one following the next. Each carbon has at least two hydrogen atoms attached to it except for the first and last carbon, which have three. Due to its exclusive carbon-hydrogen makeup and the fact that it only has single molecular bonds, it can be classified as a straight-chain alkane.

The compound is also easily represented visually. When drawn as a Kekulé structure, it is a line of six carbons, each of which has four line-bonds. Hydrogens surround the central carbon chain so that the condensed structure is written as CH3CH2CH2CH2CH2CH3. It is a simple line with five segments.
Physical Properties
This compound is stable at room temperature, and most commonly occurs as a colorless liquid. It has a melting point of roughly -139.54°F (-95.3°C), a boiling point of 154.04°F (67.8°C), and it’s molar mass is 86.18 grams per mole (g/mol). Hexane is also a non-polar molecule, which means that it is not soluble in water.
How It’s Extracted

Hexane occurs in a couple of different places in nature, but is usually most readily available in petroleum deposits. This is often why gasoline contains it in high concentrations. When petroleum and petroleum-containing oils are mined and refined, chemists are often able to isolate the compound, which can then be purified and sold commercially.
Popularity as a Solvent

One of the most popular uses is as an industrial cleaner or degreaser. It is very effective breaking molecules down and separating fats and lipids from other substances. From time to time it may be found in household cleaning products, too, but it is usually most common in solvents designed for use on heavy machinery or in places where a lot of space needs to be cleaned somewhat quickly. The solution isn’t normally very expensive, either, which is often a factor.
In Oil Processing
Many types of plants and vegetables are treated with this chemical in order to extract their oils and proteins for use in other products. Soybeans, peanuts, and corn are some of the most common. The compound is often able to break these foodstuffs down very efficiently, and the oils that result are typically ready to be repackaged and either sold or used in manufactured foods with very little additional treatment.
Other Common Uses
As good as it is at breaking compounds down, hexane can also be good at helping things stick together, particularly when used in conjunction with other non-water soluble compounds. It is often found listed as an ingredient in leather and shoe glue, for instance, and is sometimes used in roofing or tiling adhesives, too.
Controversy and Risks
Hexane is generally believed to be toxic or at least harmful when inhaled, and there have been instances of workplace injury and even death when people have spent long hours each day exposed to its fumes. This is most common in factories where oil extractions, industrial cleaning, or certain manufacturing operations take place. High exposure can cause skin irritation, dizziness, and nausea that progressively worsen over time.
There have also been questions about hexane residues that linger in vegetable oils, particularly when these show up in food products available in the general marketplace. Some health advocates argue that the presence of this chemical is unacceptable and dangerous, while others say that it is benign and shouldn’t be a cause for alarm. In most cases the amounts that actually end up in food are very, very small — but still, not a lot is known about how the compound behaves once ingested. Most of the toxicity studies that have been conducted have focused on inhalation and topical skin exposure.
AS FEATURED ON:
AS FEATURED ON:











Discussion Comments
Hexane is used in the production of Rapeseed oil and also in what most people think is a "good for you" oil, Sunflower oil. Avoid both and also Pomace olive oil; it uses the same process.
It is not safe. Just like some people are allergic to peanuts, the residue of hexane is not safe is food products. It caused a stroke like symptoms in me last year and I am still recovering from it.
Most people are aware of the additives that are put in food but few are aware of the dangers of the chemicals used in the processing of food and that does not have to be put on the ingredient list because it is only used in processing.
Are "concretes" and other essential oils derivatives extracted with hexane safe or toxic? Can they truly be considered "organic"?
Soy is a hoax! It is not a health food; it is highly dangerous. It's been falsely marketed as "health food" since the 90's for Monsanto's profit. Do your research! Denial ain't a river in Egypt.
My husband and I bought a neat looking gas can at a yard sale a few months ago. We took it to our lake house for decoration. A friend pointed out that it said Hexane and could be dangerous. What do you think about?
Soy contributes to the production of neutoroxins in the digestive tract. There is no hexane left in a food after it is used within it.
I habitually use soy milk, and have recently started using the soy 'meat' products. News about the use of the neuro-toxin Hexane in the processing of some vegetarian foods is extremely disturbing. I will be checking the 'ingredients' of these products before purchasing any soy products in future.
this is a very informative article for the novice future user of this product. thank you wise geek.
Post your comments