What Is Surface Tension?
Surface tension is the force of cohesion exhibited by the molecules of a liquid. This force allows the surface of a liquid to resist, to a degree, outside forces applied to it. It is this resistance which allows for example, a paper clip to float on the surface of a glass of water even though the paper clip has a density greater than the water. The tension varies, depending on the liquid, and with other factors such as temperature.
The tendency of a liquid to exhibit the property of surface tension arises from the attraction that the molecules of a liquid have for each other. Within the liquid, each molecule is surrounded by other molecules, and each attracts every other surrounding molecule equally, resulting in a net force of zero. The molecules at the surface of the liquid, however, are not surrounded in all directions by other molecules. They pull more strongly on the molecules that are near them, creating surface tension.
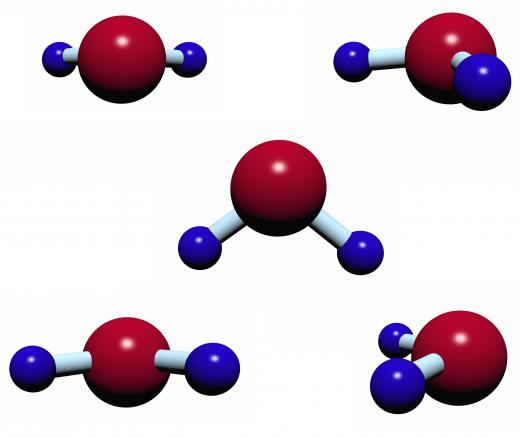
Water droplets form because of surface tension, and it also allows air to form bubbles in a liquid. As molecules on the surface of a liquid pull on other molecules at the surface, a volume of liquid in space will tend to form a sphere and, in the absence of gravity, water droplets form perfect spheres. This occurs because a sphere is the shape that has the smallest possible surface area for a given volume. When two small droplets collide in the absence of gravity, the attraction of the molecules for other molecules tends to cause the droplets to combine into a larger droplet. This tendency can sometimes be observed under standard Earth gravity as well.
The elongated shape of raindrops is due to the pull of gravity on the droplets. Surface tension tends to be a very weak force, so the droplets are easily deformed by gravity or other forces like centripetal or centrifugal forces. Some insects and even animals have adapted to take advantage of this force, however, weak though it may be. Water bugs and small lizards called basilisks actually rely on the surface tension of water to walk on it without sinking.
The surface tension of a liquid, usually expressed as dynes per centimeter, is the amount of force required to break the surface of a particular liquid over a linear distance of one centimeter. A dyne is a unit of energy or force defined as the amount of energy required to accelerate one gram of mass at a rate of one centimeter per second squared. One dyne is also equal to the International Standard (SI) unit of the micro-newton.
AS FEATURED ON:
AS FEATURED ON:








Discussion Comments
I remember that pepper experiment myself, but I also remember my science teacher would sometimes make heavier things float just by putting them on paper and slowly lowering the paper into the water. If everything worked right, the objects would not sink because of the surface tension of water. They weren't floating like a boat, either. These things should have sunk like rocks, but the water surface tension was strong enough to hold them up.
I remember the first demonstration of the surface tension water I ever saw used pepper, a bar of soap and a kitchen pan filled with water. The teacher put a white piece of paper on the bottom of the pan so we could see the trick better.
She sprinkled some black pepper very carefully on the surface of the water, and it floated. She said the reason the pepper didn't sink to the bottom was surface tension. If that surface tension was broken, the pepper would do some interesting things. She then dipped an edge of the soap bar into the water and all of the pepper floated away quickly.
She said the soap broke the water's surface tension, and the pepper was carried away by the "skin" of the water. I tried the same experiment at home every day for a week. My mother wanted to kill me, I think.
Post your comments