What Is the Shielding Effect?
The term "shielding effect" refers to a decrease in attraction between electrons and the nucleus in an atom. Electrons are highly attracted to the nucleus, because they have a negative charge and the nucleus contains protons, which have a positive charge. When additional electrons are present in different orbits, the electrons repel each other slightly. This repulsion force works against the nucleus attraction force, decreasing the attraction between the electrons and the nucleus.
Electrons in an atom can be found in several orbits. The first orbit can contain two electrons in total. Additional orbits contain various numbers of electrons, with the outermost orbit known as the valence orbit. The electron shielding effect mainly applies to the valence electrons. Electrons found in the inner orbits will shield the attractive force from the nucleus.

The positions of the electrons explain how much shielding occurs. Electrons in the first orbit, called S electrons, are shielded the least because they are closest to the nucleus. Electrons in the second orbit, the P orbit, are shielded slightly more. Electrons in the third orbit, the D orbit, are shielded more than in the P orbit. Therefore, the more electrons in an atom, the further the distance from the nucleus and the greater the decrease in attraction.
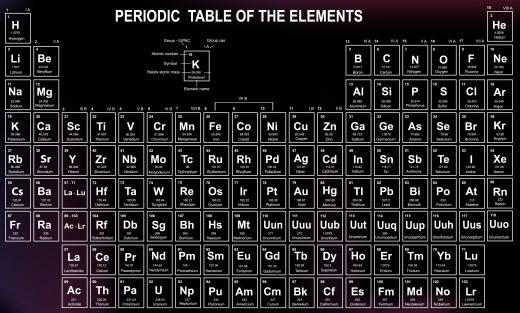
The strength of shield effects can be estimated using the periodic table. The configurations of elements in the table provide specific periodic trends, one related to this effect. Each row of the table refers to a new electron level, with the bottom rows having the most levels and the top row only one level. This means that the effect is greater on elements found at the bottom of the table.
A strong shield effect will influence the ease in which electrons can be removed, which is known as ionization energy. Electrons on the first orbit are very difficult to remove, because they must get past electrons on all other orbits. Electrons on the outer orbits are very easy to remove in chemical reactions and processes because there are no other electrons blocking the way. When an atom has one less electron than usual or one more electron, it is called an ion.
Shielding is an important chemical property, and in metals it has important solid state functions. This effect is used in metals to reduce electrostatic fields in semiconductors. It also reduces the magnitude of any electric fields produced inside the metal. Electric fields have a charge and distance, and the greater the shield, the shorter the field.
AS FEATURED ON:
AS FEATURED ON:









Discussion Comments
@OeKc05 – Yes, I have. I actually own a laptop radiation shield, because I don't want to risk overexposure.
I've read that the electromagnetic field that my computer puts off can cause everything from low sperm count to depression. I bought this pad that goes under my laptop, and I use it whenever I actually sit the computer in my lap.
Of course, if I'm at my desk, I can always just put it right on there instead of on my legs. However, there are times when I have nowhere but my lap to put the thing, so I keep the pad in my laptop case at all times.
Has anyone heard of EMF shielding? Scientists seem to think that we are getting too much radiation from our computers, so they have come up with things to protect us from it.
This shielding effect definition brought back memories from science class! It's been so many years since I actually thought about protons and electrons.
It's amazing how stuff like this sticks with you in the back of your mind, though. As I read this article, everything started to sound so familiar.
Post your comments