What are Aromatic Hydrocarbons?
 Mary McMahon
Mary McMahon
Aromatic hydrocarbons are a class of chemical substances that are characterized by having molecular structures that are called benzene rings. The chemically simplest is benzene, and the structure of this hydrocarbon lent its name to the benzene ring. Many of these hydrocarbons are toxic, and they are unfortunately among the most widespread of organic pollutants.
A hydrocarbon is any chemical compound that contains only hydrogen and carbon. Some hydrocarbons may also contain traces of impurities, as is the case with some aromatic hydrocarbons. In these cases, the impurities caused distinctive scents, leading chemists to term these compounds “aromatic.” In fact, not all smell; the scent was thought to be linked to the benzene ring, but it is actually caused by impurities. The name as stuck, however, as often happens in science even after new information on a topic has emerged, to minimize confusion.
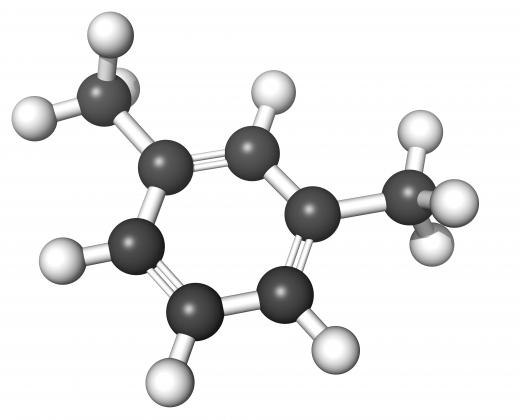
A benzene ring is a molecular structure that is created when six carbon atoms connect with each other in a linked ring. Each carbon atom has four electrons; two electrons link up with neighboring carbon atoms, while one goes to a hydrogen atom. The fourth is what is known as a delocalized electron, meaning that it is not directly involved with a specific atom. Benzene rings are often drawn as hexagonal shapes with a circle in the middle to represent these delocalized electrons. Benzene happens to be a particularly toxic form of aromatic hydrocarbon.

When benzene rings link up, they can form a range of substances, including so-called polycyclic aromatic hydrocarbons (PAHs), or polyaromatic hydrocarbons. They are created through incomplete combustion, which is why they are so widely distributed in the natural environment. Most manufacturing facilities, for example, use combustion in their operations, potentially generating large amounts of PAHs. Some PAHs are extremely toxic, which can lead to serious problems when they have been deposited in mass amounts by human activity.
An aromatic hydrocarbon may also be known in the abbreviated form of AH or as an arene. A wide range of compounds are classified as arenes, and their potential for harm is based on their molecular structure. Many people undoubtedly interact with a range of these substances every day without being aware of it and, depending on an individual's lifestyle and activities, he may also be exposed to harmful arenes, such as benzopyrene, a PAH found in tobacco smoke and tar.
AS FEATURED ON:
AS FEATURED ON:












Discussion Comments
Wow, matthewbow07, you must have Dr. Parker for Human Anatomy II lecture as well, correct?
Can you please tell me what is the antibacterial action of aromatic hydrocarbon like xylol on E.faecalis?
anon50775, you put a circle in the middle to show the delocalization of electrons around the carbon ring.
why do we represent aromatic rings with a circle in the middle? from-pav, australia
i'm writing on petrochemicals and i need data on the value chains.
Im testing for PAH in my science fair experiment here in texas, and we found heavy amounts near busy highways but texas is in no serious danger.
im writing a paper on anorexia nervosa and have to explain why you get aromatic hydrocarbon and why blood and urine samples contained high levels of ketone bodies?
I need an aromatic product chain, and information about aromatic products and their application?
Post your comments