What is Rubidium?
 Mary McMahon
Mary McMahon
Rubidium is a metallic element located in the alkaline group on the periodic table of elements. It is used in a variety of industries, as well as in laboratories for a range of experiments. The element also has a number of radioactive isotopes that are used in research and dating of ancient fossils. Rubidium is rather reactive, making it somewhat difficult to handle since it can be unstable in oxygen rich environments. People may interact with it in the form of a component in a variety of household goods.
In appearance, rubidium is soft and silvery white. When it is burned, it emits a rich red to brown flame; this property is referenced in the element's name, which is derived from rubius, or “deepest red” in Latin. When exposed to oxygen, it will spontaneously ignite, and the element also reacts violently in water. In the air, it rapidly tarnishes as it oxidizes. The element has an atomic number of 37, and it is identified as Rb on the periodic table.
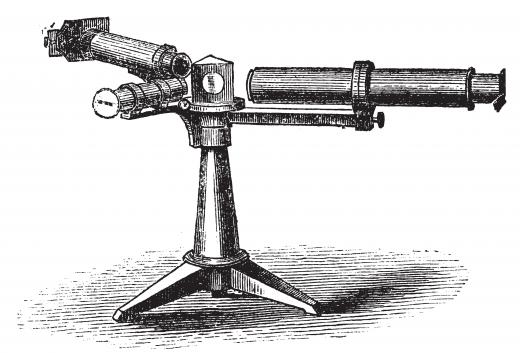
The discovery of rubidium is credited to Robert Wilhelm Bunsen and Gustav Robert Kirchhoff, who observed it in the form of a mineral compound in 1861 with the assistance of a spectroscope. This element is abundant in the Earth's crust, usually in compounds with minerals like lepidolite, leucite, and pollocite. These minerals must be treated to extract their stores of rubidium for commercial use, making the element moderately costly.
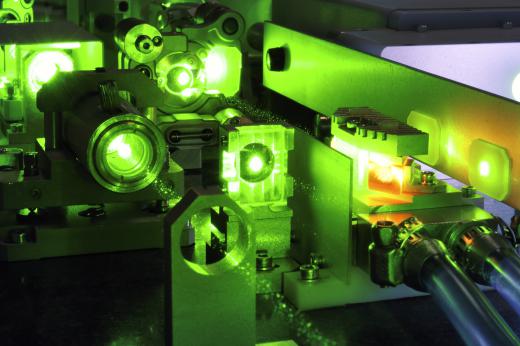
Some metal alloys include rubidium, and the element is also used in the production of glass and ceramics. Purple fireworks often get their color from this metal, and it can also be found in electron tubes, atomic clocks, and photocells. Isotopes and other forms may be used in laboratory experiments; its vapor, for example, is used in laser cooling.
Since this element is so reactive, it needs to be handled with care. It should be stored in a vacuum or in mineral oil, and it should be handled in a non-reactive atmosphere with the use of buffer gases. People who handle the pure metal are usually carefully trained in its use, and average individuals are not ordinarily exposed to it. Although rubidium does not appear to be toxic, it does not have a biological function and it may be dangerous in large amounts, so people should avoid ingesting it. Eye and face protection should also be worn when working with this element.
AS FEATURED ON:
AS FEATURED ON:










Discuss this Article
Post your comments